Navigate the road MAb
Posted on November 6, 2014
The use of monoclonal antibodies (MAbs) and MAb conjugates as biopharmaceuticals have increased over the last decade. As a result, more cost-effective, efficient, and flexible process purification solutions are of high priority for MAb manufacturers. Increasing product titers upstream can introduce challenges in downstream purification processes. With increased MAb titers, the cell culture supernatant might contain an elevated number of impurities (e.g., aggregates) that need to be separated from the target molecule.
This paper is a guide to the development of MAb purification platforms and gives an overview of GE Healthcare Life Sciences offering of process chromatography media (resins) for MAb purification processes. An introduction to highthroughput process development (HTPD) is also given, together with specific case studies of purification step development.
Introduction
Representing about 36% of the total biopharmaceutical market, with an annual sales growth rate of approximately 10%, MAbs are the single largest class of biological drugs today (1), The rapidly growing demand for MAbs has triggered a need for an elevated manufacturing capacity. As a consequence, the antibody titers in upstream cell culture processes have dramatically increased. Improved productivity upstream has put greater demand on downstream processing to address the high titers of MAbs in harvested cell culture fluids.
MAbs, as a class of molecules, exhibit many shared properties, which make them well-suited for a platform approach to downstream processing. Technology platforms (i.e., standard sets of unit operations, conditions, and methods applied to a given class of molecules) allow for efficient processing from research and development, through clinical phase trials, to the manufacturing of the final product. Downstream MAb purification platforms commonly include a protein A-based purification step followed by one or two polishing steps to remove remaining impurities (Fig 1). The use of platforms for MAb production is well-established. However, continuous improvements of these platforms are desirable as technologies are evolving. New chromatography media are introduced with enhanced features, such as higher binding capacity or better selectivity.
MAb purification toolbox
GE Healthcare chromatography media for MAb purification are all based on high-flow agarose base matrices. The capture step of the purification process is most commonly performed using protein A media such as MabSelect SuRe™ or MabSelect SuRe LX media. For the polishing steps, traditional ion exchangers such as Capto™ SP ImpRes and Capto Q media or multimodal chromatography media such as Capto adhere, Capto adhere ImpRes, or Capto MMC ImpRes are used (Table 1). To facilitate effective process development, our selection of chromatography media is available in bulk, as well as in a broad range of scalable formats for high-throughput process development.
Efficient capture of MAbs
The high selectivity, resulting in excellent purity (often 99% or more) and high yields, makes protein A-based affinity chromatography media a suitable choice for the MAb capture step. MabSelect SuRe media consists of an alkaliand protease-stabilized, recombinant protein A ligand coupled to a rigid, high-flow agarose matrix. The stability of the protein A ligand minimizes ligand leakage and allows for the use of rigorous and cost-effective cleaning procedures based on NaOH. The highly cross-linked agarose matrix of MabSelect SuRe media enables the use of high flow velocities at production scale.
To meet the needs associated with high-titer upstream processes, MabSelect SuRe LX was introduced (2). Compared to MabSelect SuRe medium, MabSelect SuRe LX offers an increased dynamic binding capacity (DBC) at a slightly longer residence time. Between 20% and 46% higher binding capacity for various MAbs has been demonstrated (3).
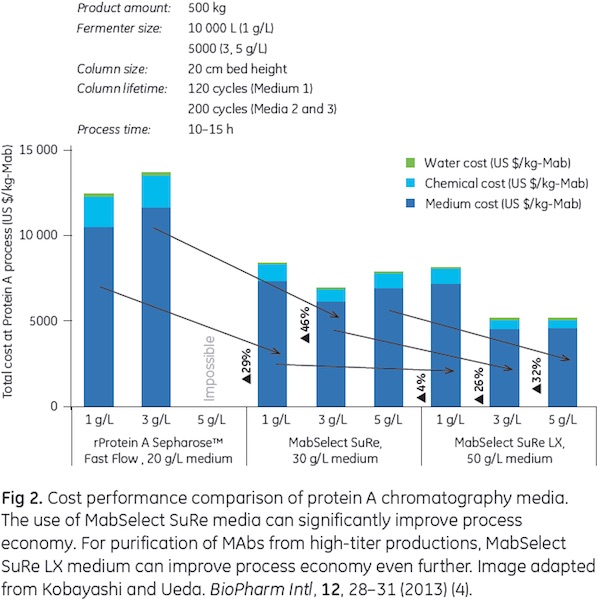
Compared with a conventional protein A medium, process economy can be significantly improved by using MabSelect SuRe media in purification of MAbs (4). When purifying MAbs from high-titer cell culture supernatants, economy was demonstrated to be even further improved by the use of MabSelect SuRe LX medium in the capture step of the downstream purification process (Fig 2).
High-resolution polishing
The polishing steps, following the capture step, can be performed in either bind-and-elute (binding) or flow-through (nonbinding) mode.
Two-step process with polishing in flow-through mode
Capto adhere and Capto adhere ImpRes media can both be used for polishing in a two-step process (Fig 3). Both media are based on the same multimodal anion exchange ligands and exhibit similar ligand densities. Hence, both media display high selectivity compared with traditional ion exchangers and are very efficient in removing remaining impurities like aggregates, host cell protein (HCP), DNA, and viruses.
The difference between the two media is the particle size. Capto adhere ImpRes medium has a smaller particle size (40 μm) than Capto adhere medium (75 μm). The smaller particle size enables higher resolution, whereas the larger particle size of Capto adhere gives the medium excellent pressure/flow properties. Hence, the choice between Capto adhere and Capto adhere ImpRes needs to be made case by case.
Three-step process with traditional ion exchangers
A three-step purification process, with two polishing steps based on one cation exchanger and one anion exchanger, is a classical way of purifying MAbs. Cation exchangers are used for the removal of HCP, protein A, aggregates, and fragments. The cation-exchange step is commonly followed by an anion exchanger (run in flow-through mode) for removal of the remaining impurities such as DNA. Suitable chromatography media for polishing in a three-step purification process are Capto SP ImpRes and Capto Q (Fig 3).
More challenging purifications
Capto adhere ImpRes can also be run in bind-and-elute mode in a two-step process for increased resolution when removing challenging impurities (5). As Capto adhere ImpRes has a smaller particle size, this medium enables higher resolution when purifying in bind-and-elute mode. The higher resolution can be beneficial, for example, for removal of challenging fragments or aggregates. A smaller particle size also means higher binding capacity and improved robustness towards residence time (Fig 4).
For certain MAbs (e.g., unstable in certain pH ranges or with a challenging impurity profile), Capto MMC ImpRes can be an alternative for use in a three-step process (6). Compared to Capto SP ImpRes, Capto MMC ImpRes has a different selectivity and a wider window of operation in terms of pH and salt concentration (Fig 5).
For more challenging aggregates, Capto adhere or Capto adhere ImpRes can be alternatives to Capto Q for use in the last step of a tree-step process. Alternative media for use in more challenging purifications are displayed in Figure 6.
High-throughput process development (HTPD)
High throughput in process development is highly beneficial in research efforts for developing optimized and robust protocols for purification of MAbs.
By the introduction of HTPD tools, significant gains in efficiency could be achieved. HTPD solutions can reduce both the required amount of sample and the time needed for development of various chromatography steps.
GE Healthcare PreDictor™ 96-well filter plates or PreDictor RoboColumn™ units, prefilled with BioProcess™ chromatography media, are suitable for efficient highthroughput screening of both different chromatography media and different chromatographic conditions during process development. Defined conditions can be verified and further optimized using small-scale columns (Fig 7).
More information on HTPD can be found in the handbook High-throughput Process Development with PreDictor Plates (code no. 28-9403-58).
Case studies
The objective of these case studies was to evaluate different strategies for polishing of MAbs. Both two- and three-step processes were evaluated. Three different MAbs were used in these studies. The purification targets were set to aggregate < 1%, yield > 90%, and HCP < 20 ppm.
Development of the polishing step (flow-through mode) of a two-step process
Screening of flow-through conditions for MAb1 using Capto adhere ImpRes was initially conducted in PreDictor 96-well filter plates. Further optimization was performed in smallscale columns using a design-of-experiment (DoE) approach.
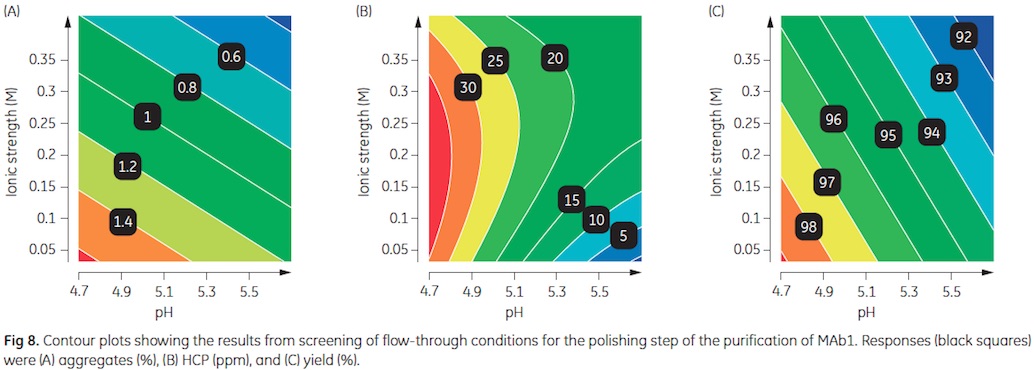
Conditions screened were pH 4.7 to 5.7, 0.032 to 0.42 M ionic strength (IS), and 60 to 100 g/L sample load. Responses monitored were aggregates, HCP, and product yield. Data were evaluated with MODDE™ software.
The results are illustrated in Figure 8. The contour plots display low aggregate content at high pH and high IS (Fig 8A), low HCP content at high pH and low IS (Fig 8B), and highest yield at low pH and low IS (Fig 8C).
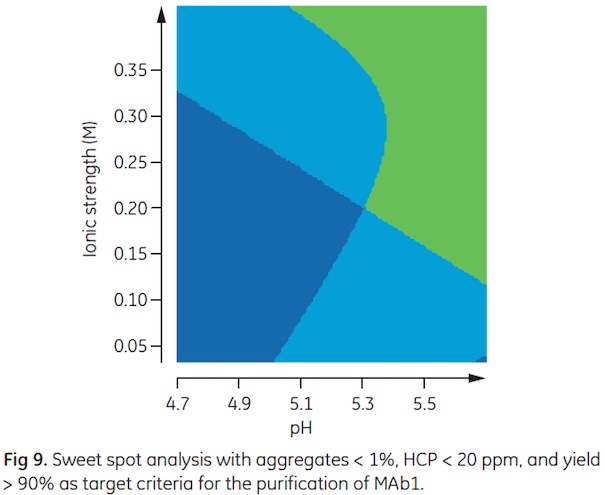
As the optima do not coincide, a sweet spot analysis was performed with the target values given above. The green area of the sweet spot plot shown in Figure 9 represents the conditions were all three criteria are met. Run conditions, further verified in small-scale columns, were pH 5.7, 0.23 M IS, and 80 g/L sample load. The predicted run conditions where all three target criteria were met correspond well with the verification experiment (Table 2).
Examples where Capto adhere medium, based on the larger particle size, have been used in polishing steps can be viewed in Application notes 28-9509-60 and 28-9078-89 (7, 8).
Development of the polishing steps of a three-step process
Screening of binding conditions for MAb2 using Capto SP ImpRes was performed in PreDictor 96-well plate format.
Conditions screened were pH 4 to 8 and 0 to 250 mM NaCl. A contour plot from the screening of static binding conditions (SBC) is displayed in Figure 10. The red area corresponds to the highest SBC and the blue to the lowest.
For binding, pH 5 and 5.5 were identified as suitable. Based on the results from the binding study, elution conditions were selected (illustrated by white arrows in Fig 10) and validated in small-scale columns. A comparison between elution at pH 5 and pH 5.5 showed similar yield, but aggregate
clearance was improved at pH 5 (data not shown). From the gradient elution experiment, a step elution protocol was developed for Capto SP ImpRes at pH 5.0 (Fig 11). The results show that aggregates were efficiently removed with high MAb yield (Table 3).
To further reduce the impurity levels (e.g., HCP levels), a Capto Q flow-through step was added to the process (Fig 12). More details about development of a Capto Q step can be found in application note 28-9037-16 (9). The results from Capto SP ImpRes and Capto Q polishing steps are summarized in Table 3.
Capto adhere ImpRes in bind-and-elute mode
Because of poor resolution between the monomer and aggregates, MAb3 could not be purified in flow-through mode on Capto adhere ImpRes, and hence, bind-and-elute mode was evaluated. Screening of binding conditions for Capto adhere ImpRes was performed in PreDictor 96-well filter plates. Conditions screened were pH 4 to 8 and 0 to 300 mM NaCl. The highest SBC was obtained at high pH and low salt concentration (Fig 13).
Studies of different elution strategies were done in smallscale columns, with a pH gradient from pH 7.8 to 4, with or without addition of salt. In this case, the results showed that addition of 100 mM NaCl in the elution buffer resulted in improved aggregate removal. As shown in Figure 14,
aggregates eluted in the tail of the peak. At 90% yield, the aggregate content was reduced from 1.2% to 0.3 % when run in bind-and-elute mode.
Based on the results from gradient elution, a step elution protocol was developed. Binding was performed at pH 7.8 and elution by addition of 45 mM NaCl and reducing pH to 5.4 (Fig 15). Strip was performed at pH 3.5.Analyses of the elution pool showed 0.5% aggregates at 90% yield, low HCP content, and a protein A level below detection limit. Results are summarized in Table 4.
Analyses of the elution pool showed 0.5% aggregates at 90% yield, low HCP content, and a protein A level below detection limit. Results are summarized in Table 4.
Virus clearance in both flow-through and bind-elute mode
Viral clearance in MAb polishing steps has been demonstrated with model viruses. In a study of Capto adhere run in flowthrough mode at two different conductivities, the medium was shown to effectively remove both minute virus of mice (MVM) and murine leukemia virus (MuLV) at high conductivity (Table 5).
To evaluate virus clearance by using Capto adhere ImpRes in bind-and-elute mode, pooled fractions from a MAb capture step using MabSelect SuRe were used. The pool was spiked with stock solutions of MVM and MuLV. MAbs in the spiked sample were purified using the step-elution protocol
described for MAb3 (Fig 15). The elution pools were analyzed for virus titer by end-point titration. The results showed a reduction factor of 5 logs for both viruses. For MVM, residual infectivity was detected in the strip fraction, whereas for MuLV, residual infectivity was inactivated in the low-pH elution step.
Conclusions
A toolbox comprising modern chromatography media is useful in the development of effective purification platforms for MAbs. Protein A media, such as MabSelect SuRe LX, enables efficient purification of MAbs to high purity and yield from high-titer harvested cell culture fluids. The alkalistabilized protein A ligand permits the use of NaOH for rigorous and cost-effective cleaning operations. A selection of high-resolution Capto ImpRes chromatography media can be used in subsequent polishing steps.
Multiple examples of development of efficient MAb purification processes have been demonstrated in this paper. Multimodal chromatography media such as Capto adhere and Capto adhere ImpRes offer the possibility of two-step purification processes. Traditional ion exchangers such as Capto SP ImpRes and Capto Q media can be combined for efficient removal of remaining impurities in tree-step processes. For more challenging purification tasks, alternative use of Capto MMC ImpRes and Capto adhere ImpRes can be applied.
For efficient process development, GE Healthcare’s selection of chromatography media is available in a broad range of scalable formats for HTPD applications. Let our expertise and experience in the development of MAb processes support you in navigating your path to success.
References
1. Aggarwal S. What’s fueling the biotechnology engine? Nat Biotechnol 29, 1083-1089 (2011)
2. Thillaivinayagalingam, P., Reidy, K., Lindeberg, A., Newcombe, A.R. Revisiting Protein A Chromatography. BioProcess International 10, 36-39 (2012)
3. Application Note: Dynamic binding capacity study on MabSelect SuRe LX for capturing high-titer monoclonal antibodies. GE Healthcare 28-9875-25, Edition AA (2011)
4. Kobayashi, S and Ueda, Y. Comparing Protein A Resins for Monoclonal Antibody Purification. BioPharm Intl, 12, 28-31 (2013)
5. Application note: Polishing of monoclonal antibodies using Capto adhere ImpRes in bind and elute mode. GE Healthcare, 29-0273-38, Edition AA (2013)
6. Application note: Polishing of monoclonal antibodies using Capto MMC ImpRes in bind and elute mode. GE Healthcare, 29-0373-49, Edition AA (2013)
7. Application note: High-throughput screening and optimization of a multimodal polishing step in a monoclonal antibody purification process. GE Healthcare 28- 9509-60, Edition AC (2012)
8. Application note: Optimization of loading conditions on Capto adhere using design of Experiments. GE Healthcare 28-9078-89, Edition AA (2007)
9. Application note: Process-scale purification of monoclonal antibodies – polishing using Capto Q. GE Healthcare, 28-9037-16, Edition AB (2012)
For local office contact information, visit
www.gelifesciences.com/contact
GE Healthcare Bio-Sciences AB
Björkgatan 30
751 84 Uppsala
Sweden
GE, imagination at work, and GE monogram are trademarks of General Electric Company.
ÄKTA, ÄKTApilot, ÄKTAprocess, AxiChrom, BioProcess, Capto, HiTrap, HiScreen, MabSelect SuRe, PreDictor, Sepharose, and Tricorn are trademarks of GE Healthcare companies. MODDE is a trademark of Umetrics AB. RoboColumn is a trademark of Atoll GmbH.
© 2013 General Electric Company — All rights reserved.
First published Dec. 2013
All goods and services are sold subject to the terms and conditions of
sale of the company within GE Healthcare which supplies them. A copy
of these terms and conditions is available on request. Contact your
local GE Healthcare representative for the most current information.
GE Healthcare UK Limited
Amersham Place
Little Chalfont
Buckinghamshire, HP7 9NA
UK
GE Healthcare Europe
GmbH, Munzinger Strasse 5
D-79111 Freiburg
Germany
GE Healthcare Bio-Sciences Corp.
800 Centennial Avenue, P.O. Box 1327
Piscataway, NJ 08855-1327
USA
GE Healthcare Japan Corporation
Sanken Bldg., 3-25-1, Hyakunincho
Shinjuku-ku, Tokyo 169-0073
Japan
Related Topics and Keywords
mAb, MAb conjugates, MAb purification, MabSelect SuRe LX media, MabSelect SuRe™, manufacturing, monoclonal antibodies, Protein A media
Subscribe to our FREE newsletter and WEBINAR UPDATES
We will not sell or give your information to a third party. See our Privacy Policy