A sequential dosing approach for the Zydis® freeze dried orally disintegrating tablet, by L.Grother, L. Garrett, R. McLaughlin
Posted on September 11, 2014
OBJECTIVES
The Zydis® freeze dried dosage form commonly utilises a matrix solution or suspension that contains the active pharmaceutical ingredient (API) and any other excipients such as colours, flavours or sweeteners. This is dosed into blister pockets and frozen by passing through a liquid nitrogen freeze tunnel; before being freeze dried to produce the orally disintegrating tablet (ODT).
Temperature sensitive APIs need to be processed at low temperatures and therefore non-gelling matrix formers are required. However, such formulations are prone to surface defects, such as cracks and nodules which may adversely affect patient compliance.
The aim of this work was to determine whether sequentially dosing two separate matrix solutions, with different gelling characteristics, into the same blister pocket could achieve a single ODT of acceptable quality.
METHODOLOGY
Two prototype Zydis® formulations were manufactured containing non-gelling and gelling matrix formers, these were held at 5°C and 23°C respectively. A 300mg aliquot of the non-gelling formulation was dosed into blister pockets, rapidly frozen and then freeze dried. To assess the sequential dosing approach, 150mg of the non-gelling formulation was first dosed into blister pockets followed by 150mg of the gelling formulation. These samples were then processed under the same freezing and drying conditions as the non-gelling only formulation. A total of 400 blister pockets of each formulation were dosed. Prior to dosing, the viscosity of each mix was measured using a Haake VT550 viscometer. Disintegration testing was carried out using USP methodology. Dispersion testing was carried out in 600ml of purified water at 37°C (±0.5°C).
Additional formulations were manufactured using the same processing conditions and formulation components with the addition of blue dye to one of the layers to help visualise the bilayer effect.
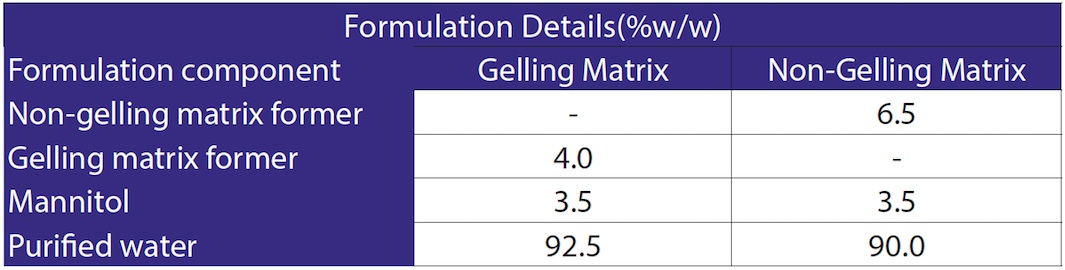
The formulation details are included in Table 1.
Table 1. Formulation Details
RESULTS AND DISCUSSION
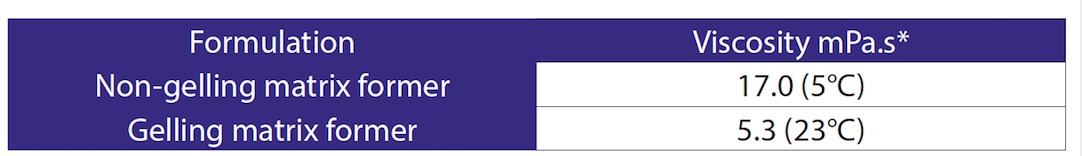
The viscosity results demonstrated that the non-gelling matrix formulation had substantially higher viscosity than the gelling matrix formulation. This was due to the lower dosing temperature. Both formulations were of a fluidity that allowed them to be accurately dosed. Viscosity data is given in Table 2.
Table 2. Viscosity Data * Reading taken at shear rate of 500s-1
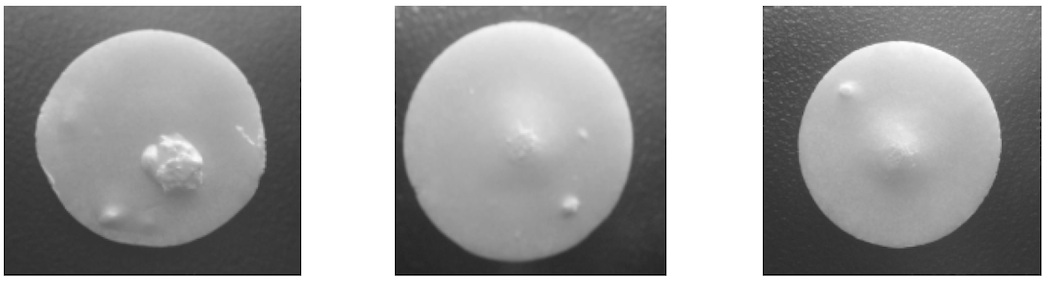
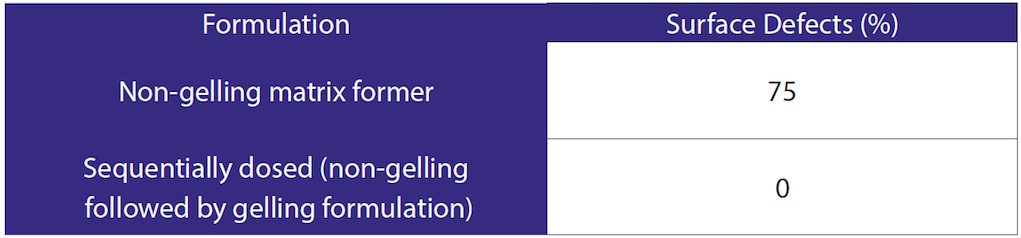
When dosed on its own, the non-gelling formulation was susceptible to creating surface defects in the freeze dried tablet. No defects were found on inspection of the tablets with the non-gelling and gelling formulations dosed sequentially. Examples of surface defects are shown in Figure 1. Inspection results are given in Table 3.
Figure 1. Example of Tablets with Surface Defects
Table 3. Inspection Results
The use of blue dye enables observation of the two discrete formulation layers demonstrating that there was little potential for interaction of the two layers prior to freezing of the tablet. Photographs of the tablets highlighting the two layers are shown in Figure 2.
Figure 2. Photographs of Sequentially Dosed Tablets
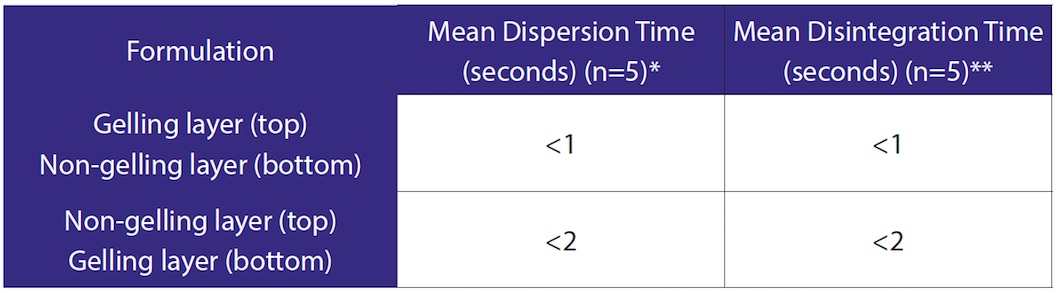
A key attribute of the Zydis® ODT is its rapid dispersion time; typical dispersion times can be as little as 3 seconds (compared to compressed ODT formulations which display dispersion times of up to 30 seconds). Dispersion and disintegration (USP) testing was carried out on the sequentially dosed tablets and the results given in Table 4 show that the sequential dosing approach has had no negative impact on the dispersion times, regardless of the order of dosing each formulation. The disintegration times comply with the FDA Guidance for Industry: Orally Disintegrating Tablets (2008).
Table 4. Dispersion and Disintegration Test Results
*600ml Purified water at 37°C ±0.5°C
** 750ml Purified water at 37°C ±2.0°C
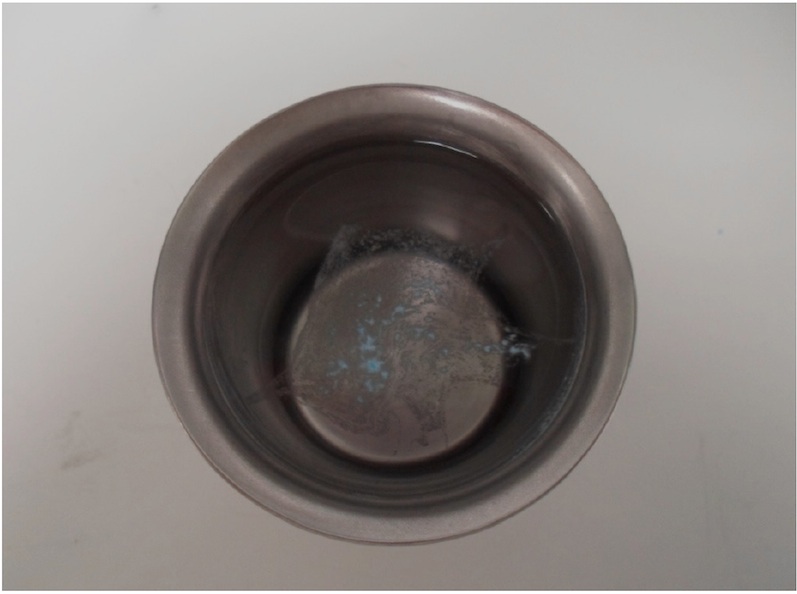
The instant dispersion of the tablet when in contact with water is shown in Figure 3.
Figure 3. Dispersion Test in 600ml Purified Water, < 2 seconds at 37°C
As well as reducing surface defects found when dosing temperature sensitive API, the sequential dosing approach has the scope to overcome numerous formulation challenges. For example, sequential dosing may also serve to:
– Keep separate two incompaptible APIs in combination products.
– Keep separate API and any incompatible excipients.
– Add a buffering layer to the tablet that may otherwise be incompatible with the API.
CONCLUSIONS
This study confirmed that a sequential dosing approach proved effective in the manufacture of Zydis® product with acceptable appearance and dispersion time. The method was successful in reducing the surface deformations seen with the use of non-gelling matrix former alone.
The sequential dosing approach could also be used to address formulation challenges such as the need to include incompatible APIs or excipients in the same formulation, thus potentially opening the Zydis® ODT technology to a number of new applications.
REFERENCES
1. H.Seager, Drug-delivery Products and the Zydis® Fast-dissolving Dosage Form”, J.Pharm.Pharmacol.,50(1998) 375-382.
© 2013 Catalent Pharma Solutions. All rights reserved.
To Learn more, go to: http://www.catalent.com/index.php
Related Topics and Keywords
acceptable quality., API, Catalent, colours, excipients, flavours, freeze dried orally disintegrating tablet, matrix solution, ODT, oral disintegrating tablet, sequential dosing approach, single ODT, suspension, sweeteners., Zydis
Subscribe to our FREE newsletter and WEBINAR UPDATES
We will not sell or give your information to a third party. See our Privacy Policy