Development of a High-Performance Integrated and Disposable Clarification Solution for Continuous Bioprocessing
Posted on April 18, 2017
Mike Collins and Peter Levison, PhD
Pall Life Sciences
Current bioprocesses combine fed-batch cell culture with batch-wise downstream processing steps. To achieve integrated upstream and downstream continuous manufacturing, the industry has been in need of a continuous cell separation and clarification solution for bioprocess fluids from bioreactors. The Cadence Acoustic Separator from Pall Life Sciences provides this solution, with continuous first-stage clarification without the need for filter media in a scalable, single-use format with no negative impact on product attributes. The Cadence Acoustic Separator delivers cost and time savings through reductions in buffer volumes, filter preparation time, and depth-filter requirements with stable performance and higher yields over longer processing times.
LIMITATIONS OF EXISTING TECHNOLOGY
As the biopharmaceutical industry slowly but surely moves toward adoption of integrated continuous manufacturing, suppliers such as Pall are driven to develop solutions that specifically enable continuous downstream unit operations to be linked together.
Efficient solutions for continuous harvesting of biologic drug substances from cell culture processes have been lacking until recently, however. Biologic active pharmaceutical ingredients (APIs) must be separated from the particulate contaminants in cell culture bioprocess fluids before they can be clarified. This clarification step removes solids and turbidity to generate a clear harvest cell culture fluid.
Currently, depth filtration and centrifugation are the methods most commonly used for clarification of bioprocess fluids. Filters, however, can present challenges for bioprocesses commonly observed today that involve solutions with high cell mass. And filters eventually become clogged and must be cleaned or replaced, leading to facility downtime. Centrifuges can handle higher cell mass feeds, but higher gravitational forces that can be harmful to living cells are typically required.
BENEFITS OF ACOUSTIC WAVE SEPARATION
Recognizing the challenges associated with switching from batch to continuous operations, Pall Life Sciences sought a solution that would not require either primary depth filtration or centrifugation. And, in early 2015, the team purchased an exclusive license to cutting-edge acoustic wave separation technology (AWS) developed by FloDesign Sonics.
Rather than use a physical barrier or shear forces to remove cell debris and other contaminants, the Cadence Acoustic Separator clarifies bioprocess f luids through generation of forward propagating acoustic waves and the ref lection of backward propagating acoustic waves. As cells enter the counter-current f low channel, the acoustic forces trap them from the flow. As the number of trapped cells increases, they begin to clump and agglomerate. Eventually, the clump becomes large enough that gravitational forces overcome the drag forces and cause the cells to sediment.
An important point is that clarification using AWS technology occurs as a continuous process in a closed system that does not result in any significant rise in temperature. As a result, there is no impact on protein purity. The AWS technology also provides high yields with predictable and reproducible purity profiles over a wide range of cell densities at both process development and commercial scales for various types of biologic products, including recombinant therapeutic proteins and monoclonal ntibodies.
As is the case for current depth filtration processes, clarification with the Cadence Acoustic Separator is typically followed by a polishing step through a small depth filter to remove any remaining turbidity. Elimination of the large depth filtration step, however, means that 75% of the filter area is no longer needed. Although sizing of the depth filter for polishing after clarification with the Cadence Acoustic Separator is dependent on the characteristics of the feedstock, a 3- to 10-fold reduction in depth filter area can be achieved. The result is significant reductions in cost, tank sizes, and overall operational footprint.
SCALABLE TECHNOLOGY
The first Cadence Acoustic Separator is a bench-scale unit for use during process development and optimization studies. It consists of a 1-in. x 2-in. acoustic chamber and operates with a typical process feed f low rate (single pass) of 3.6 L/h. In the acoustic chamber’s dimensions, the 2 in. is the path length from transducer to the ref lector. Two to four acoustophoretic separation chambers are connected in a series to enhance clarification efficiency, even for the challenging cell culture feed streams produced using current fed-batch and perfusion technology.
The Cadence Acoustic Separator is constructed using a modular design, allowing for clarification of bioprocess fluids from bioreactors with volumes up to 2,000 L.
CASE STUDY: CLARIFICATION OF MONOCLONAL ANTIBODY (MAB) IN CHINESE HAMSTER OVARY (CHO) CELL CULTURE FLUIDS
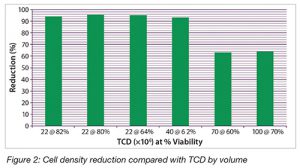
To demonstrate the effectiveness of the Cadence Acoustic Separator for clarification of bioprocess fluids with a range of properties, CHO-based mAb cell culture fluids with different total cell density (TCD) values ranging from 22 to 40 x 106 cells/mL and cell viabilities ranging from 60 to 82% were processed. The prototype PD scale Cadence Acoustic Separator (1-in. x 1-in. chamber) with a three-stage (series) element was used to perform the study. The same operating conditions were maintained for all of the separations by setting the acoustic control to automatic mode. The results can be seen in Figure 2.
At ultrahigh cell densities, the Cadence Acoustic Separator showed very promising results, including for TCD values as high as 100 x 106 cell/mL. It is expected that, with minor optimization of process conditions and product design to handle higher masses, equivalent performance should be possible even at these very high cell densities.
OPTIMIZATION OF THE SECONDARY DEPTH-FILTRATION STEP
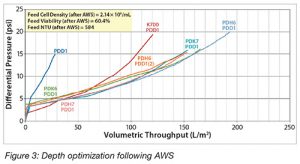
To determine the optimum conditions for the polishing of clarified fluid following acoustic wave separation, AWS-purified feeds from a number of different mAb cell culture processes were evaluated. The results in Figure 3 show that dual-stage depth filtration outperformed single-stage filtration.
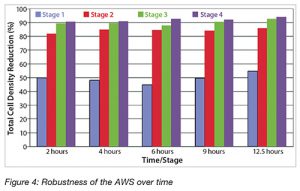
The robustness of the Cadence Acoustic Separator was also evaluated to determine its suitability for continuous processing. In this study, the Cadence Acoustic Separator (four-stage) was operated for an extended 13.5-h run to demonstrate continuous process capability. The feed was taken from a 25-L fed-batch cell culture process and had a TCD of 23.5 x 106 cells/mL, a turbidity of 2,466 nephelometric turbidity units (NTU), a spin-down turbidity of 85.5 NTU, a PCM value of 6.0%, a cell viability of 82%, and a mAb concentration of 1.7g/L.
The Cadence Acoustic Separator was connected to a depth filter, which was continuously operated at a low flux rate of 15 L/ m²/h (LMH). Throughout the entire run, the process remained very stable, as can be seen in Figure 4.
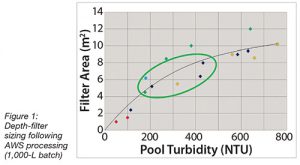
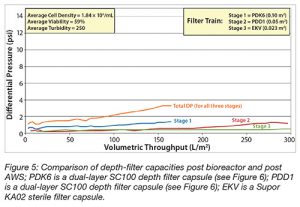
Finally, the required depth filter capacity following AWS clarification was evaluated for a mAb cell culture bioprocess fluid. As can be seen in Figure 5, the depth-filter capacity was enhanced more than four-fold compared with that required directly post bioreactor (data not shown). Specifically, the filter area was reduced from 0.55 m2 to 0.15m2.
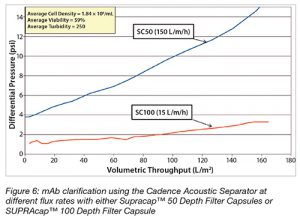
Furthermore, stable operation of the Cadence Acoustic Separator was achieved at both high and very low flow rates (Figure 6). The capacity was, notably, three times greater at a flux of 15 LMH compared with that at 150 LMH (using the same grade of filter at different scales).
PERFORMANCE SUMMARY
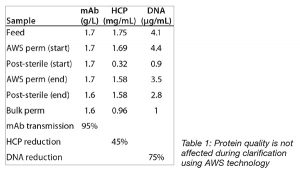
Most important, clarification of a mAb cell culture fluid using the Cadence Acoustic Separator did not affect the quality of the antibody (Table 1) and proceeded with high transmission of the antibody through the system.
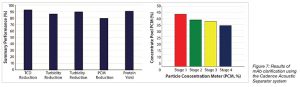
The antibody was obtained in high yield with significant reduction of cell debris and other contaminants, as shown in Figure 7.
A CUTTING-EDGE SOLUTION
The Cadence Acoustic Separator from Pall Life Sciences is the most cutting-edge solution for continuous clarification of cell culture bioprocess fluids. The robust, single-use system allows for quick setup and stable long-term operation. The reduction of buffer volumes and filter media requirements translates into significant cost savings, and eliminating the need to prepare large filters also saves time. Most important, the Cadence Acoustic Separator provides clarified proteins in high yield without any impact on product attributes and can be used for a wide variety of biologic APIs at process development and commercial scales.
FEATURED PRODUCT
Cadence™ Acoustic Separator (CAS)
Streamline Clarification with Acoustic Wave Separation Technology
As the biopharmaceutical industry moves toward continuous processing for more flexible, cost-efficient manufacturing in multiproduct facilities, new methods are needed for the continuous harvesting of biologic drug substances from cell culture processes. One challenge to this process is that host cells and their debris must be removed from harvested cell culture fluid (HCFF) so that biologically active pharmaceutical ingredients (APIs) can be clarified.
Currently, depth filtration and centrifugation are the most common methods for clarification of bioprocess fluids. Filtration, however, can be challenging for solutions with high cell mass and may result in high product losses. Moreover, filters eventually become clogged and must be cleaned or replaced, leading to facility downtime. Centrifuges can handle higher cell mass feeds but typically require higher gravitational forces that can be harmful to living cells by increasing the amount of cell debris and feed contaminants that need removal further downstream. Thus, like depth filtration, centrifugation can present issues of maintenance and low product yield in some applications.
To overcome these issues, Pall Life Sciences offers the Cadence Acoustic Separator (CAS) system — a cuttingedge acoustic wave separation (AWS) technology developed by FloDesign Sonics (FDS) and exclusively licensed by Pall. In June 2015, Pall Corporation signed an exclusive license for this disruptive separation technology from FDS for cell culture clarification both in fed-batch and perfusion applications. The technology enables purification of bioprocess fluids without using a primary depth filter or centrifuge.
Mild Conditions Without Clogging:
The Cadence Acoustic Separator system is ideal for biopharmaceutical applications because it causes no significant increase in temperature or damage to cells or proteins. It is robust and reliably provides high yields with predictable and reproducible performance over a wide range of cell densities at both the process development and clinical manufacturing scales.
AWS technology applies acoustic forces across a countercurrent flow of bioprocess fluid to generate threedimensional standing waves that trap cells at their nodes, leading them to agglomerate and eventually sediment as they lose buoyancy. With CAS, cells can be continuously removed in a closed system without centrifugation or primary depth filtration, thus streamlining this challenging step in the biologics manufacturing process within a small operating footprint.
High Yields and Reduced Costs:
Compared with large-scale depth filtration, the Cadence Acoustic Separator system typically allows for the reduction of 75% of the required filtration area, resulting in significant cost savings and a reduction in tank sizes and overall operational footprint. The process also is highly reproducible, providing HCCFs with consistent purity profiles. It has proven to be effective for clarification of several types of biologic products — including recombinant therapeutic proteins and monoclonal antibodies — regardless of the variability in particulate concentrations and cell culture density, turbidity, and viability.
CAS is a clear choice for high–celldensity, fed-batch Chinese hamster ovary (CHO) cell culture clarification. It provides scalable, continuous clarification that can be directly linked to primary downstream capture chromatography. CAS normalizes batchto-batch cell culture harvest at the first stage of clarification, thus leading to consistent secondary polishing requirements, lower safety margins in sizing filtration area, and substantial gains in process economics.
www.pall.com/continuous-questions
The original version of this article was published as part of BioProcess International’s June 2016 special issue sponsored by Pall Life Sciences, and is reproduced with permission from BioProcess International.
Related Topics and Keywords
acoustic wave separation technology, AWS, batch-wise downstream processing, bioprocess fluids, bioreactors, Cadence Acoustic Separator, combine fed-batch cell culture, continuous cell separation, continuous first-stage clarification, Disposable Clarification Solutions, High Performance Intergrated Solutions, integrated upstream and downstream continuous manufacturing, Mike Collins, Pall Life Sciences, Peter Levison, without filter media
Subscribe to our FREE newsletter and WEBINAR UPDATES
We will not sell or give your information to a third party. See our Privacy Policy